|
PRENATAL INVESTIGATIONS
The purpose of prenatal
diagnosis is detection or exclusion of a hereditary disease or congenital
defect in utero. The option of an elective abortion of affected
pregnancies can help parents at risk of having affected children to have a
normal offspring in the future.
Hereditary skin diseases
if diagnosed in early pregnancy may help very much to minimize social,
psychological, and financial states. It may help in planning of having
normal babies instead of repeated abortions, miscarriage or malformed
deliveries.
If the fetus is aborted
after a positive diagnosis, a careful post-mortem examination should be
carried out in order to confirm the diagnosis and search for other
possible congenital abnormalities.
Methods
of Diagnosis
Amniotic fluid and its
cells are used for diagnosing a variety of metabolic diseases,
morphological, biochemical and cytogenic.
Ultrasonography: is a
powerful tool for the detection of central nervous system and skeletal
disorders.
Fetoscopy: this is a
technique that involves the insertion of a fibro-optic endoscope into the
pregnant uterus.
Amniocentesis: is a
convenient and relatively safe method of obtaining amniotic fluid and its
cells for morphological, cytogenetic and biochemical investigations. This
test is usually performed at 16 weeks of gestation.
Fetal skin biopsy: helps
in the detection of morphological or immuno-histochemical abnormalities.
Enzymatic assay
DNA Examination: DNA test
helps to detect any genetic abnormalities.
Cyto-diagnosis :
Examination of
scrapings from the lesion or the contents of the vesicles can help in
reaching diagnose of certain skin diseases. Cytological smears should be
taken from early fresh lesions.
Thin smear on a clean
glass slide is left to dry, stained with Wright‘s stain, hematoxylin
and eosin or Giemsa stain. This can be examined with the low power and
then by the high power to have an idea about the content of the smear.
GENERAL INVESTIGATIONS
Tzank Test
Cytological examination
from the floor of a bulla is used to confirm diagnoses of bullous
diseases.
In most bullous
eruption the smear will show only inflammatory cells.
In pemphigus numerous
acantholytic cells with large nuclei and condensed cytoplasm are found.
Herpes simplex, zoster
and varicella lesions: the smear shows large, multi-nucleated and
mono-nucleated giant cells and ballooning degeneration of the nuclei.
Diascopy
Diascopy is
a simple procedure
which will sometimes provide useful additional information for diagnosis
of certain skin diseases such as in lupus vulgaris that shows
distinctive yellowish, reddish brown apple jelly nodules.
A glass slide or a
clear plastic tongue depressor is pressed firmly on the lesion. The
temporary exclusion of blood clearly reveals the presence and sometimes
the probable nature of dermal changes.
Examination of skin
scrapings
This is usually used for
the diagnosis of fungal lesions. Scraping is taken of the lesions of the
scalp, intertriginous areas, feet or other areas. The skin is cleaned by
alcohol swab and left to dry.
Scrape the area with a
scalpel or the edge of the slide on a clean slide. Add one drop of 10-20
per cent of KOH or SMS preparation. Hyphae and spores appear as oval
bodies and refractile against the background of cells and debris.
|

Fig. 9. Diascopy
|
Confirmation is usually
by culture of the scrapping on special media such as Sabouraud‘s agar
medium.
Blood picture
Different skin diseases
show local or systemic blood changes. Therefore blood picture may be of
help to reach a diagnosis and may be indispensable in certain dermatoses.
Meanwhile, not every dermatological case is in need of a list of
laboratory tests that may be a burden and might bother the patient leading to
loss of confidence in the physician.
Blood picture may show
the following in certain skin diseases:
Neutrophilia
Neutrophilia may
accompany the following skin diseases:
-
Infections, e.g.
erysipelas, carbuncle.
-
Inflammatory disorders
including pustular or inflammatory psoriasis, erythroderma, and pyoderma
gangrenosum.
-
Systemic malignancy
(leukemia).
-
Reaction to systemic
steroid therapy.
Eosinophilia
Eosinophilia is common in
the following diseases:
-
Atopic disorders,
especially asthma and eczema.
-
Allergy to food or drugs.
-
Parasitic infestations:
worms (intestinal or systemic), scabies.
-
Collagen vascular
disease, polyarteritis nodosa, dermatomyositis.
-
Bullous disorders:
dermatitis herpetiformis, pemphigus, and pemphigoid.
-
Erythema neonatorum.
-
Malignancy, especially
Hodgkin‘s disease and eosinophilic leukemia.
Lymphocytosis
Lymphocytosis may be
present in the following diseases:
-
Viral infections,
especially exanthemata and infectious mononucleosis.
-
Bacterial infections:
tuberculosis, syphilis, brucellosis, and typhoid.
Erythrocyte Sedimentation
Rate (ESR)
ESR is usually
non-specific test. A raised ESR is usually due to an increased aggregation
of red cells due to an abnormality of plasma proteins, notably an increase
in plasma fibrinogen associated with the acute or chronic phase reaction.
Some causes that raise
ESR:
-
Physiological: pregnancy,
menstruation, advancing age.
-
Infections.
-
Inflammatory disorders,
e.g. vasculitis.
-
Systemic lupus
erythematosus (SLE).
-
Tissue destruction.
-
Malignant neoplasms.
-
Paraproteinaemias.
-
Polycythaemia.
Serum Protein Estimation:
this test is used in certain diseases as systemic lupus, hypoproteinemia.
Antinuclear Antibody
(ANA)
May be present with:
-
Collagen vascular
disease, especially SLE.
-
Chronic liver diseases.
-
Hashimoto‘s
thyroiditis, thymoma, myasthenia gravis.
-
Pernicious anemia.
-
Tuberculosis.
-
Leprosy.
-
Diffuse pulmonary
fibrosis.
-
Lymphoma or other
malignancy.
-
Ulcerative colitis.
SEROLOGICAL
TESTS
These tests are indicated
in certain diseases such as syphilis and non-venereal treponemas mainly
Pinta and Bejel.
Liver function tests
Diseases of liver may
manifest with internal and cutaneous manifestations.
Hormonal essay: is an
important line in investigating certain skin diseases especially those
associated with endocrine dysfunction.
Cryoglobulins tests
This test shows
precipitation of proteins when cooled which redisolves again when heated.
Cryoglobulins are not
present in normal individuals.
Skin diseases that show
positive test:
Purpura
Cold sensitivity cyanosis
Raynaud‘s disease
Lupus erythematosus
Lymphgranuloma venerum
Leg ulcers
Cutis marmorata.
Technique:
In a warm 10 ml. syringe,
venous blood is collected and the serum is separated at 37*C, then cooled
in a refrigerator to 5*C. A gelatinous precipitate forms that redisolves
when rewarmed.
Porphobilinogen Urine
Test
This test is specific for
acute intermittent porphyria. It is simple and
valuable in screening
patients suspected for porphyria.
Technique
5 ml of freshly voided
urine is mixed with 5 ml of Ehrlich‘s reagent and mixed with 10 ml
aqueous saturated sodium acetate. The solution is then extracted with
equal volume of chloroform. Porphobilinogen and urobilinogen form a red
aldehyde compound with Ehrlich‘s reagent.
Skin biopsy
Skin biopsy is an
important procedure to confirm an accurate diagnosis for a suspected skin
lesion.
Skin
Tests
Skin tests are introduced
into the skin by a variety of techniques to study pharmacological and
immunological reactions under controlled conditions. Such tests are
extremely valuable, but details of the type of test and the time at which
it is read must correspond to the pathological process under
consideration.
Interpretation of the
relevance of tests, either positive or negative, must always be correlated
with the clinical picture.
Severe systemic reactions
may occur after the use of standard testing solutions, therefore
anti-shock measures as oxygen, adrenaline and hydrocortisone injections
should always be at hand when skin tests are performed.
Intradermal tests
are much more sensitive than percutaneous methods (the tested allergen
is 10- to 100-fold more diluted), but they have a lower specificity.
Intradermal testing is usually reserved for venom and penicillin
allergy testing when percutaneous tests are negative but there is high
clinical suspicion of allergy.
Stinging-Insect
Hypersensitivity. Adults who present with a history of a systemic
reaction to insects (e.g., bee, yellow jacket, hornet, wasp, fire ant)
should be evaluated with allergy skin tests. Children who present with
only dermatologic manifestations of a systemic reaction are not at
substantially increased risk for future anaphylaxis and do not need
allergy skin tests. Management of sensitive patients may include
education, avoidance measures, self-administered epinephrine, and
allergen immunotherapy.
Drug Allergy.
Reliable allergy tests for drugs are available only for penicillin and
local anesthetics. In many patients with a history of penicillin
allergy, the simplest course is to prescribe an antimicrobial agent
that does not contain a beta-lactam ring. In patients with a history
of penicillin allergy who have a strong indication for use of a
beta-lactam antibiotic, penicillin skin tests can be helpful.
These results
suggest that penicillin can be given safely to patients with negative
intradermal skin tests to penicillin. Patients with positive
penicillin skin tests may be at increased risk for drug reaction, but
the specificity of intradermal testing is low.
Percutaneous
testing
Several types of
skin testing instruments are available for percutaneous skin testing.
Each brand of instrument has its own sensitivities and specificities.
Positive-control skin tests (histamine) and negative-control skin
tests (diluent) are essential for correct interpretation of skin test
reactions. About 15 minutes after the application of allergen to skin,
the test site is examined for a wheal and flare reaction. A positive
skin test reaction (typically, a wheal 3 mm greater in diameter than
the negative control reaction, accompanied by surrounding erythema)
reflects the presence of mast cellbound IgE specific to the tested
allergen.
Allergy to airborne
substances (i.e., allergic rhinitis and asthma) is typically evaluated
using a panel of percutaneous skin tests for about 40 allergens. A
number of the most commonly used allergenic extracts for skin tests
are now standardized . Percutaneous skin testing has
been used to test for food allergy; however, it is less reliable for
evaluating food allergy than for evaluating reaction to airborne
allergens.
The specificity for
food allergen tests is generally low, partly because of cross
reactions between some food groups (e.g., legumes). Negative reactions
to suggested food allergens on percutaneous tests make a diagnosis of
true food allergy unlikely in most cases; however, the poor
specificity of these tests precludes a definitive diagnosis of food
allergy based on positive test results alone. A double-blind food
challenge should be considered when more clinical certainty is needed
in diagnosing a serious food allergy.
|
Positive
and negative skin controls are important in establishing
reliable results. Antihistamine medications can interfere with
skin testing and should be stopped beforehand. Intradermal testing is more sensitive than
percutaneous methods but much less specific. Its use is
restricted to testing for allergy to insect stings or
penicillin. In cases where skin testing is not available or
desirable, laboratory assays for IgE antibodies to specific
allergens may be used. These assays are generally less sensitive
than skin testing methods.
|
There are several
types of specific allergy tests.
1- Immediate-type hypersensitivity
(IgE)
skin tests are typically used to test for airborne allergens, foods,
insect stings, and penicillin. Immediate-type hypersensitivity also
can be evaluated through serum IgE antibody testing called
radioallergosorbent testing (RAST). Immediate-type
hypersensitivity skin testing is most commonly used in the diagnosis
of allergic rhinitis, allergic asthma, food allergy, penicillin
allergy, and stinging-insect hypersensitivity. Skin testing can be
performed by the percutaneous route (diluted allergen is pricked or
scratched into the skin surface) and by the intradermal route
(injection of allergen within the dermal layer).
2- Delayed-type hypersensitivity skin
tests (patch-type skin tests) are commonly used in patients with
suspected contact dermatitis. Some common allergens for patch testing
are rubber, medications, fragrances, vehicles or preservatives, hair
dyes, metals, and resins. This review focuses on immediate-type
hypersensitivity skin testing and serum IgE antibody testing.
PATCH TESTS
Patch tests are usually
used to detect contact sensitizers of the delayed hypersensitivity type.
Patch test is easy to apply and more safe than other skin tests.
Patch testing proves only
that the patient has a contact sensitivity to a specific contactant, but
this does not necessarily mean that this
substance in the patch test is the only that can cause the reaction but
there may be other substances that may cause such reaction.
Precautions:
Precautions should be
considered to have correct and safe interpretation.
-
Antihistamines intake:
patients using antihistamines especially the long acting or those on
systemic steroids .The test should be postponed till the effect of these
is minimal which may take few days or weeks. Antihistamines usually have
no appreciable effect on delayed hypersensitivity patch tests.
-
Acute eczematous
lesions: re-exposure with more antigens in the test may cause more
flare-ups of the lesions especially in children and hypersensitive
patients.
-
Dilution of the
testing substance: The substance applied in the patch test should not be
irritant substance and this is why the substance should be diluted and not
applied in its full strength as in cosmetics or other sensitizers.
Different Patch Tests
-
Open patch test - this
test is used in testing the plant oleoresins.
Method:
Acetone extracts of the
plants, weeds or trees are applied on the skin surface. The area is kept
dry and the result is noted after 48 hours.
-
Provocative patch test
- this test is used for detection of sensitivity of neomycin, penicillin
and benzocaine.
Provocation of the open
patch test is maintained by application of 10 per cent of sodium lauryl
sulfate to the test area for one hour. A significant reaction may appear
when the patch test is done.
-
Vapor patch test - this
test is applied for volatile substances such as perfumes.
Apply the vapor or gas
to the skin surface under a small glass cup tapped into the skin for 48
hours.
-
Mucous membrane patch
test - this test is used for local sensitizing agents for the mouth as
mouthwashes, nicotine and toothpaste.
A small suction cup
containing the test material is applied to the mucous membrane of the
lip and kept for one hour. Control is essential in this type of test.
-
Photo patch test - this
test is used for detection of photosensitizing substances such as
phenothiazine, sulfa, and photosensitizing plants.
Patch test is done in the
ordinary way for 48 hours where the area is exposed to ultraviolet
radiation and then read again after another 48 hours. Control test area is
necessary for exclusion of false positive or negative reactions.
Technique of Ordinary
Patch Test
Patch testing is available nowadays ready, where the antigen can be applied
directly on the testing area.
The sites for the tests are usually on the back or inner arms. If these
are not available the diluted substance can be applied on gauze and
covered by elastoblast.
The reaction may be
detected after 30 minutes as in contact urticaria. These are usually read
at 48-72 h and again up to 1 week.
If pruritus, pain or
irritation occurs, patch testing should be removed and mild steroid may be
applied to the area.
Interpretation of Patch
Test
The result of patch test
is interpreted as follows:
1+: Erythema only.
2+: Erythema and papules.
3+: Erythema, papules and
small vesicles.
4+: Large vesicles, bullae,
and severe local reaction besides erythema.
False Reactions
False positive and
negative reactions are common in patch testing. This is due to different
factors:
-
Low concentration or
insufficient amount may give false negative.
-
High concentration and
increased amount may cause local irritation.
-
Improper testing as
the substance is not fresh, or presence of impurities in the testing
substance or the occlusion was not complete.
-
The patient is under
antihistamine or systemic steroid will give false negative reaction.
Intradermal Tests
Site used for testing:
the injection is made into the superficial layer of the dermis in the
flexor surface of the forearm.
Needle used: through a
fine bore (26 or 27) needle with its bevel pointing upwards.
Quantity of the solution
injected: the quantity, which may be injected, varies from 0.01 to 0.1 ml
and routinely 0.05 ml is usually sufficient.
Technique
The test is accomplished
by putting the skin under tension with the fingers of one hand: the other
hand inserts the needle attached to the tuberculin syringe containing the
test material.
Interpretation
of the test
Time of reading the test:
The optimal time for reading the reaction naturally varies with the
pharmacological agent or the type of immunological reaction. Most such
tests are read at either 15-20 min or at 48 h, but it may be important to
read the tests at other times, e.g. at 4-12 h or after 4 days.
Control solution: The
test solution must always be compared with a control solution injected in
a comparable site at the same time.
A positive test may be
taken as one that is significantly different from the control. Assessment
of what is significant is difficult and varies with the enthusiasm of the
tester.
The response can be
observed at 15 minutes, for example, after an injection of histamine or after
immediate-wheal allergy tests, is a wheal with a surrounding flare.
The wheal is a more
accurate measure than the flare. When the test is read at 48h, for
example, the tuberculin reaction, the sizes of the indurated papule and of
the erythematous reaction should be observed.
The measurement of a
wheal
is usually made by diameter. The size of the weal is not directly
proportional to the dose of the active agent but varies also with the
total volume of fluid injected. For accurate quantitative observations
weal diameters below 4 mm or above 15 mm cannot be relied upon.
Precautions before doing
the test:
-
Antihistamines:
Antihistamines may greatly inhibit the immediate wheal tests. For the very
long-acting drugs as terfenadine or astemazole, this effect may last as
long as 3 weeks.Antihistamines
interfere with the development of the wheal and flare reaction and
should be stopped before immediate-type skin testing. First-generation
antihistamines may be stopped two to three days before testing, but
the newer, second-generation antihistamines can affect skin testing
results for three to 10 days or longer.
Medications with antihistamine properties, such as anticholinergic
agents, phenothiazine, and tricyclic antidepressants, also should be
discontinued before skin testing. Histamine H2-receptor
antagonists (e.g., cimetidine [Tagamet], ranitidine [Zantac]) have a
limited inhibitory effect; these medications may be stopped on the day
of skin testing.1
Inhaled and short-term systemic corticosteroids generally do not
significantly suppress the wheal and flare reaction of immediate-type
skin tests.
-
Corticosteroids:
Moderate-to-large doses of corticosteroids in contrast may somewhat
inhibit patch tests although smaller doses, e.g. Prednisone 10 mg daily,
are not necessarily a contra-indication to testing. Steroids do not greatly
inhibit the immediate weal tests.
-
Antishock measures
should be at hand.
PRICK TEST
This is a modification of
the intradermal test and is convenient for much routine allergy testing.
The intradermal injections of prick test solutions may be dangerous.
Technique: A small
quantity of the test solution is placed on the skin and a prick made
through it with a sharp needle. This should be superficial and not
sufficient to draw blood.
|
The size of the weal and
flare
are measured after 15 min.
|
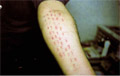
Fig. 11. Scratch
test
|
SCRATCH
TEST
The scratch test
resembles the prick test.
Technique: A linear
scratch about 1 cm long, but not sufficient to draw blood, is made through
the epidermis. This test gives less reproducible results than the prick
test.
Modified prick test
This test is slightly
more sensitive than the ordinary prick test, but gives no more
reproducible results.
Technique: A drop of the
test solution is placed on the skin. A needle is then inserted very
superficially and almost horizontally into the skin and lifted to raise a
tiny tent of epidermis.
Immediate wheal tests
Indications:
-
These tests are used
for detecting IgE antibodies.
-
The passive
transfer test may be used to detect circulating IgE, but is not
recommended because of the risk of serum hepatitis or AIDS.
-
They are principally
used in the assessment of hay fever and asthma and have a limited place
in the management of atopic dermatitis.
-
They are
disappointing in the diagnosis of urticaria.
-
False-positive and
false-negative reactions are common.
-
Rast
& Elisa Tests
Rast (Radio-Allergosorbent Test) and ELISA (Enzyme-linked Immunosorbent
Assay) are alternative methods to detect and measure circulating
antibodies.
Although widely used
in the past, serum measurement of the total IgE level is unhelpful in
the diagnosis of allergy. Of more clinical use are assays for specific
IgE antibodies to suspected allergens.
Assays for IgE
antibodies specific to common airborne and food allergens are readily
available. IgE antibody tests for venom and drugs have less clinical
utility and are not routinely used. RAST was the first widely employed
method of detecting IgE antibodies in blood that are specific for a
given allergen.
hat include a reference curve
calibrated against standardized IgE are preferred. It is important to
select a reliable laboratory to perform RAST testing.
In general, RAST and
other laboratory methods for IgE testing are highly specific but
somewhat less sensitive than percutaneous tests. Results of laboratory
testing for food-specific IgE are generally poor, even less helpful
than those for percutaneous skin testing.
RAST or other
laboratory testing is typically considered when skin testing is
inconvenient or difficult to perform. Most primary care physicians do
not have immediate access to a clinical skin testing laboratory, so
RAST may be easier to obtain. Some patients cannot undergo skin
testing because of skin disease that would obscure wheal and flare
results (e.g., extensive atopic dermatitis) or because they cannot
stop taking medications that suppress the skin test response. In cases
of life-threatening allergy (e.g., anaphylaxis), laboratory testing is
sometimes used as a proxy result, keeping in mind its limited
sensitivity.
Percutaneous testing
can help establish the correct diagnosis and identify the offending
allergens (pollen, mold spores, dust mites, cockroaches, or household
pets). Allergen avoidance measures often are difficult to implement
and costly. After specific testing, avoidance measures can be targeted
to allergens to which the patient is known to be allergic.
Allergen
immunotherapy is another option in refractory cases of allergic
rhinitis not amenable to the usual control measures. Like allergen
avoidance, it can involve a lot of labor and expense. Specific allergy
testing can identify patients likely to benefit from immunotherapy and
provide guidance about which allergens to include in the therapy
regimen. Allergen immunotherapy may be especially beneficial when
avoidance and medications no longer control the patient's symptoms.
Several closely related variants are marketed (e.g.,
modified RAST, Quidel QuickVue One-Step Allergen screen, Pharmacia
Immunocap). Quantitative assays test.
RAST correlates well
with skin testing. It is justified in testing very young children, and
with allergens associated with risk on prick testing (e.g. drugs).
-
Oral provocation tests
These tests must be
carried out with care and is only valuable if the test is properly
controlled and the patient is co-operative and well motivated. The
administration of a drug, food or chemical by mouth may sometimes be
called for to confirm the diagnosis of an eruption or to establish its
exact cause.
Indications:
-
Drug eruption. To
determine the cause of a drug eruption or to isolate one from a number
of drugs or ingredients of a compound drug. It may be a valuable method
of proving the cause of a fixed drug eruption but should rarely, if
ever, be used if the reaction has been of a generalized or acute nature.
It is applicable only
when the drug given and the dose chosen are unlikely to provoke a severe
reaction in the patient.
-
Atopic eczema
-
Chronic or recurrent
urticaria.
-
Food allergy. The
re-introduction of specific foods, or additives such as:
Tartrazine, benzoates
and anti-oxidants one at a time, is an established part of exclusion,
elimination and challenge diets. It is important that the role of the
suspect food is subsequently confirmed by reintroducing it in a
disguised form to avoid identification by the patient.
-
Elimination and exclusion
tests
These tests are used to
detect the blamed food, additives or beverages that are suspected to cause
dermatitis. Exclusion of the suspected material for few days and observing
the skin lesion may give an indication of the effect of the eliminated
material.
-
Wood‘s light
This is an ultraviolet
lamp with Wood‘s filters, which produces a wavelength about 3650 Â.
Wood‘s light is an important investigative tool in diagnosis and
treatment of specific skin diseases.
Wood‘s lamp may be used
to help in the diagnosis of the following lesions:
Fungal infections: Tinea
capitis caused by Microsporon species gives bright blue-green
fluorescence. It should be noted that Tricophyton tonsurans and
Tricophyton violeceum types of ringworm do not give fluorescence.
Erythrasma: gives a coral-red fluorescence.
Pityriasis versicolor
lesions when examined in a dark room with Wood‘s light appear as sharply
accentuated lesions.
Bacterial infections:
Pseudomonas pyocyanea gives a yellowish-green color due to pyocyanin.
The acne bacillus causes
a coral fluorescence in the follicles possibly due to porphyrin
production. Erythrasma gives coral- red or pink-orange fluorescence.
Detection of pigmentary
disorders:
Wood‘s lamp can be used
to determine the depth of melanin in the skin, since variations in
epidermal pigmentation are more apparent under Wood‘s lamp than under
visible light. Wood‘s light accentuates contrast between pigmented and
non-pigmented skin and separates hypopigmented from totally non-pigmented
areas as in vitilligo and albinism.
Detection of porphyrins:
Porphyrins in urine when
examined in dark field by Wood's light, gives red color or pinkish orange.
Porphyrins in feces, blister fluid in porphyria lesions, the teeth in
erythropoietic porphyria and blood protoporphyria give also the same
fluorescence.
Erythropietic porphyria
can be also diagnosed by the fluorescence of red cells.
Tetracycline: deposits in
the growing enamel teeth of children that produces a typical yellow color
of the teeth under Wood‘s light illumination.
Malignant
tumors of the skin especially squamous cell carcinoma gives bright-red fluorescence.
Miscellaneous:
Medications, industrial
compounds and other fluorescent materials can be detected specifically by
Wood‘s light.
REFERENCES
-
Auerbach AD.
Diagnosis of diseases of DNA synthesis and repair that affect the skin
using cultured amniotic fluid cells. Semin Dermatol 1984; 3: 172-84
-
Ahlestedt S.
Eriksson N, Lindren S, et. Al., Specific IgE determination by RAST
compared with skin and provacation tests in allergy diagnosis with birth
pollen. Timothy pollen and dog epithelium allergens. Clin Allergy 1974;
4:131-140.
-
Boyd AS, Neldner KH.
The isormorphic response of Koebner. Int. J. Dermatol 1990; 29: 131140.
-
Committee on
Provocative Food Testing. Identification of food allergies. Ann Allergy
1973; 31: 375-92.
-
Caplan RM. Medical
uses of Wood‘s lamp. J Am Med Assoc 1967; 202: 123 5.
-
Pepys J. Skin tests.
Br J Hosp Med 1984; 32: 120-4.
-
Dutz W, Kohout E.
Dermatologic diagnosis by using the hemocytometer and the dental broach.
Int J Dermatol 1982; 21: 410-11.
-
Diagnosis. London:
Royal College of Obstetricians and Gynaecologists, 1984: 147-58.
-
Eady RAJ, Rodeck CH.
In: Rodeck CH, Nicolaides KH, eds. Prenatal Elias S. Use of fetoscopy
for the prenatal diagnosis of hereditary skin disorders. In: Gedde-Dahl
T, Jr, Whepper.
-
Farmer ER, Hood AF,
eds. Pathology of the Skin. London: Prentice Hall International, 1990.
-
Gosden CM, Ross A,
Eason PJ. In: Sandler M, ed. Amniotic Fluid and its Clinical
Significance. New York: Marcel Dekker, 1981: 37-103.
-
Harrison PV. A
guide to skin biopsies and excisions. Clin Exp Dermatol 1980; 5: 235-43.
-
KD, eds. Prenatal
Diagnosis of Heritable Skin Diseases. Current Problems in Dermatology.
Basel: Karger, 1987: 1-13.
-
Kaback MM. In:
Rodeck CH, Nicolaides KH, eds. Prenatal Diagnosis. London: Royal College
of Obstetricians and Gynaecologists, 1984: 1-12.
-
Lehman CW. A double
blind study of sublingual provocative food testing: a study of its
efficacy. Ann Allergy 1980; 45: 144-9.
-
McKee PH. Pathology
of the Skin. Philadelphia: Lippincott, 1989.
-
Marks R, Dawber
RPR. Skin surface biopsy: an improved technique for the examination of
the horny layer. Br J Derm 1971; 84: 117-23.
-
Mills OH, Kligman
AM. The follicular biopsy. Dermatologica 1983; 167: 57 63.
-
Polani PE.
Incidence of developmental and other genetic abnormalities. Proc Roy Soc
Med 1973; 66: 1118-19.
-
Patrick AD.
Inherited metabolic disorders. Br Med Bull 1983; 39: 378-85.
-
Serup J.
Quantification of weal reactions with laser Doppler flowmetry. Allergy
1985; 40: 233-7.
-
Seltzer JM.
Correlation of allergy test results obtained by IgE RAST and
prick-puncture methods. Ann Allergy 1985; 54: 25-30.
-
Boyd AS, Neldner
KH. The isomorphic reponse of Koebner. Int J Dermatol 1990; 29: 401-10.
-
Rothman S.
Physiology and Biochemistry of the Skin. Chicago:
University of Chicago
Press, 1954.
-
Caplan RM. Medical
uses of Wood‘s lamp. J Am Med Assoc 1967; 202: 123-5.
-
Gilchrest BA,
Fitzpatrick TB, Anderson RR et al. Localization of melanin pigment on
the skin with Wood‘s lamp. Br J Dermatol 1977; 96: 245-8.
-
Gottlieb PM,
Stupniker S, Askovitz J. The reproducibility of intradermal skin tests:
a controlled study. Ann Allergy 1960; 18: 949-60.
-
Lever WF,
Schaumburg-Lever G. Histopathology of the Skin 7th edn. Philadelphia:
Lippincott, 1990.
-
McKee PH. Pathology
of the Skin. Philadelphia: Lippincott, 1989.
-
Pepys J. Skin
tests. Br J Hosp Med 1984; 32: 120-4.
-
Ahlstedt S,
Eriksson N, Lindgren S et al. Specific IgE determination by RAST
compared with skin and provocation tests in allergy diagnosis with birch
pollen. Timothy pollen and dog epithelium allergens. Clin Allergy 1974;
4: 131-140.
-
Ackerman AB.
Histologic Diagnosis of Inflammatory Skin Diseases. Philadelphia: Lea
& Febiger, 1978.
-
Farmer ER, Hood AF,
eds. Pathology of the Skin. London: Prentic
Hall International, 1990.
-
Harrison PV. A
guide to skin biopsies and excisions. Clin Exp Dermatol 1980; 5: 235-43.
-
Eady RAJ. Fetoscopy
and fetal skin biopsy for prenatal diagnosis of genetic skin disorders.
Semin Dermatol 1988; 7: 2-8.
-
Eady RAJ, Rodeck
CH. In: Rodeck CH, Nicolaides KH, eds. Prenatal Diagnosis. London: Royal
College of Obstetricians and Gynaecologists, 1984: 147-58.
-
Gosden CM, Ross A,
Eason PJ. In: Sandler M, ed. Amniotic Fluid and its Clinical
Significance. New York: Marcel Dekker, 1981: 37-103.
-
Auerbach AD, Adler
B, Chaganti RSK. Prenatal and postnatal diagnosis and carrier detection
of Fanconi anemia by a cytogenetic method. Pediatrics 1981; 67: 128-35.
-
JAMES T.
LI, M.D., PH.D., is currently professor of medicine at the Mayo
Clinic, Rochester, Minn. He received his medical degree from Duke
University School of Medicine, Durham, N.C. He earned a doctorate in
microbiology and immunology at Duke University Graduate School,
Durham.
Lasley MV, Shapiro GG. Testing for allergy. Pediatr Rev
2000;21: 39-43.
Valyasevi MA, Maddox DE, Li JT. Systemic reactions to allergy
skin tests. Ann Allergy Asthma Immunol 1999;83:132-6.
Lockey RF, Benedict LM, Turkeltaub PC, Bukantz SC. Fatalities
from immunotherapy (IT) and skin testing (ST). J Allergy Clin Immunol
1987;79:660-77.
Carter ER, Pulos E, Delaney J, Matheson EJ, Moffitt DR. Allergy
history does not predict skin test reactivity in asthmatic children. J
Asthma 2000;37:685-90.
Li JT, Andrist D, Bamlet WR, Wolter TD. Accuracy of patient
prediction of allergy skin test results. Ann Allergy Asthma Immunol
2000;85:382-4.
Wood RA, Phipatanakul W, Hamilton RG, Eggleston PA. A
comparison of skin prick tests, intradermal skin tests, and RASTs in
the diagnosis of cat allergy. J Allergy Clin Immunol 1999;103:773-9.
Sampson HA, Ho DG. Relationship between food-specific IgE
concentrations and the risk of positive food challenges in children
and adolescents. J Allergy Clin Immunol 1997;100:444-51.
-
Sogn DD, Evans R 3d, Shepherd GM, Casale TB, Condemi J,
Greenberger PA, et al. Results of the National Institute of Allergy
and Infectious Diseases collaborative clinical trial to test the
predictive value of skin testing with major and minor penicillin
derivatives in hospitalized adults. Arch Intern Med 1992;152:1025-32.
Solley GO, Gleich GJ, Van Dellen RG. Penicillin allergy:
clinical experience with a battery of skin-test reagents. J Allergy
Clin Immunol 1982;69:238-44.
-
National Asthma Education and Prevention Program. Guidelines
for the diagnosis and management of asthma: expert panel report 2. NIH
publication no. 98-4051, Bethesda, Md., 1997:43,45.
-
Top
|


