|
CONNECTIVE TISSUE DISEASES
|
|
|
|
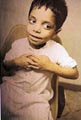
This connective tissue disorder usually appears at birth or develops at any age up to the third year. Clinical Features Skin lesions are characterized by firm, smooth, pink or flesh colored nodules, which are found on one or more fingers or toes. The thumbs and great toes are spared. The swellings, which are firmly attached to the skin, are usually on the extensor aspect or the side of the terminal phalanges. Spontaneous regression usually occurs within two years.
JUVENILE PALMOPLANTAR FIBROMATOSES This is an invasive calcifying tumor of the palms and soles with a unique histological pattern, occurring usually in young children as firm, fixed, fibrous nodules.
INFANTILE
STIFF SKIN SYNDROMES This syndrome affects infants in the first weeks of life, causing limitation of joint mobility with diffuse thickening of the joints and hyaline tissue deposit. General manifestations: gingival hypertrophy, subcutaneous nodules of the perianal, lips, and ears. Systemic manifestations: diarrhea, growth failure, recurrent infections may accompany the syndrome.
This hereditary connective tissue disorder appears in early infancy and is characterized by mild hirsutism, limitation of joint mobility and localized areas of stony-hard skin. The condition affects the deeper skin and fascia which is much thicker than normal and tends to be most pronounced in the buttocks and legs.
All the premature aging syndromes are probably inherited, though the defect may not be obvious in the first few years of life. Clinical Features Skin changes : Loss of cutaneous fat leading to atrophy and wrinkling. Canities. Hair loss. Nail dystrophy. Defective pigmentation. Poikiloderma, sclerosis and ulceration. Different syndromes associated with premature aging are:
Loss of subcutaneous fat e.g. generalized lipodystrophy.
PREMATURE
AGING SYNDROME This is an inherited syndrome that begins early after birth and is characterized by resistence to insulin. The defect is in the receptor binding, post receptor or in both. Fibroblasts respond poorly to the metabolic actions of insulin and to the actions of several other growth factors as epidermal growth factor. Clinical Features The child is abnormal at birth with low birth weight and the disease is usually fatal. Growth is generally retarded, the nutritional status remains poor and susceptibility to infection is high. The skin appears too large for the body, loosely folded at the flexures, and may be corrugated with gyrate folds on the hands and feet, which may be disproportionately large. Hypertrichosis of the forehead and cheeks. Progressive muscle wasting. The bone age is retarded and there may be metaphyseal and epiphyseal dystrophy. The nose is broad, the ears are low set and large. The eyes are widely spaced. The breasts and the penis or clitoris may be slightly hypertrophic.
WERNER‘S
SYNDROME Clinical Manifestations The earliest manifestations of the syndrome are: Graying at the temples Skin manifestations: the skin becomes tense, shining and adherent. The lower legs and feet, forearms and hands are most severely involved and to less extent the face and neck. Keratoses and ulcers: over pressure points on the feet and ankles. Pigmentation: mottled or diffuse and telangiectasia are often conspicuous on the limbs, face and neck. Subcutaneous tissue: loss of subcutaneous tissue results in a bird-like faces and thin spindly legs, which contrast with the normal or obese trunk. Joints: become fixed and there may be sclerodactaly and acral gangrene. The voice: may be high pitched and hoarse from thinning of the cords and fixation of the epiglottis. Intelligence is usually normal. Endocrine changes: small stature and hypogonadal, with sparse or absent pubic and axillary hair. Diabetes: This type is characterized by relatively low blood glucose levels and peripheral resistance to insulin. Eye changes: Cataracts develop between the ages of 20 and 35 in most cases and are usually posterior and subcapsular. Other ocular defects may occur. Malignancy: fibrosarcomas, which occur in 10% of patients. Carcinoma has developed in a chronic leg ulcer. Blood vessels: atheroma develops early. Death usually occurs in fourth to sixth decade, due to myocardial infarction or malignancy. Diagnosis The disease has characteristic clinical features with multi-systems involvement. The radiological changes are often striking. There may be calcification of arteries, ligaments, tendons and subcutaneous tissue with osteoporosis of the extremities, especially the legs.
PROGERIA This is an autosomal recessive syndrome where fibroblast survival time is decreased with increased production of hyaluronic acid that appears in urine. Clinical Manifestations General features Affected children usually appear normal at birth. In the first year the infant manifests with retarded growth. In the second year there is growth failure with reduced subcutaneous fat on the face and limbs. Prominent eyes and scalps veins, bird facial appearance, peaked nose and centrofacial cyanosis. Micrognathia and thin lips, large cranium with patent fontanels and frontal bossing. Skin manifestations Thin, taut and shiny skin in some areas but lax and finely wrinkled in others.
After several years the manifestations are: Hyperpigmentation: progressive mottled hyperpigmentation develops, most marked on exposed sites, but there is no photosensitivity. Thickened sclerotic areas: may be present on the lower trunk or thighs and in one case multiple keloids developed on the hands and arms . Nails: are usually small, thin and dystrophic. Koilonychia and onychogryphosis may occur. Teeth: The dentition is abnormal and delayed. There may be skeletal abnormalities, such as dystrophic clavicles and coxa vulga and joint contractures. Nipples: may be hypoplastic. Bone resorption: may lead to frequent fractures . Sexual maturation : is absent. Intelligence is normal. Death usually occurs in the second decade as a result of severe generalized atheroma.
Acrogeria is characterized by cutaneous atrophy and loss of subcutaneous fat particularly over the distal extremities, leading to premature aging of the extremities. Acrogeria begins at birth, where the general health and life expectancy are normal. Clinical Manifestations Short stature and low birth weight. The skin becomes dry, thin, transparent and wrinkled, especially over the hands. Poikiloderma and telangiectasia and easy bruising due to prominent veins as a result of lack of the supporting subcutaneous fat. The hands and feet may be very small. The face appears ‘pinched‘, with a hollow-cheek ‘owl-eyed‘ appearance, with a beaked nose and thin lips. Micrognathism may be present. Premature senility due to lack of subcutaneous fat. Nails may be atrophic or thickened.
The manifestation of this rare familial syndrome appears at birth. The cause is unknown, where the dermal collagen and elastin appear normal on light microscopy. Clinical Manifestations Skin manifestations Dry wrinkled skin of the hands, feet and ventral surfaces of the trunk. General manifestations The veins are unduly prominent. There may be also mental retardation, ocular defects and poor muscle tone.
Skin of diabetics may show different changes mainly: Thick, tight waxy skin, limited joint mobility, frozen shoulder and Dupuytren‘s contracture. The “prayer sign“ in which the patient tries to oppose the two palms provides an easy screening test. Retinal and renal disease due to microvascular damage. The histology of the skin changes resembles systemic sclerosis. The difference is a large collagen fibers, thickening of the capillary basement membrane and increased mucin.
Perforating dermatoses include different types of skin diseases in which some tissue protrudes from the dermis. The manifestations of this syndrome are due to defect in collagen, elastic tissue or defect in the epidermal keratinocytes. The extruded materials may show inflammatory cells, red cells, microorganisms and extracellular substances, such as mucin or altered connective tissue components.
Reactive Perforating Collagenosis The condition usually starts in early childhood. Clinical Features The primary lesion is skin colored small papules, which increase in size within one month to about half centimeter and then become umbulicated with keratinous plug. The lesions regress within two months leaving slight scarring or hypopigmentation. The lesions may recur again, can be elicited by trauma as a linear lesion, or can be produced in response to cold and regress by warming the area. Treatment Topical retinoids may reduce the number of lesions. Oral isotretinoin, methotrexate, emollient creams, topical steroids under occlusion may help some cases. Perforating serpengious elastosis The age of onset ranges from 6 to 20 years. Clinical Manifestations Small horny or umbulicated papules appear mainly on the back, sides of the neck, cheek and arms. Skin lesions may be unilateral or bilateral and symmetrical. The lesions are characteristically arranged in lines, circles or segments of circles in a serpiginous pattern. The individual papules may remain small or may enlarge slightly to assume a crateriform appearance with an elevated edge and a central plug, which may leave an area of atrophic skin surrounded by smaller papules, each with a horny plug. The lesions may persist for several years but eventually involute spontaneously to leave reticulate atrophic scars, which is liable for keloid transformation.
Colloid milium or colloid degeneration of the skin is a degenerative skin changes, characterized clinically by the development of yellowish, translucent papules or plaques on light-exposed skin and histologically by the presence of colloid in the dermal papillae. In young children, the lesions are often confined to the face, around the orbits, the backs of the hands, the back and sides of the neck and the ears, with diffuse infiltration surmounted by innumerable small papules, which may appear vesicular.
In older patients the papules are often fewer, larger and their potential distribution is much wider, although often only one or two sites are involved in each individual. Treatment CO2 laser ablation of the extensive lesions may give better cosmetic results. Superficial resurfacing by Co2 laser using topical anaesthetics as Emla creams Dermabrasion, diathermy and cryotherapy, can also give good results.
|
| Contents | << Previous Chapter | Next Chapter >> | Search |