|
DISTURBANCE OF PIGMENTATION
|
|
|
|
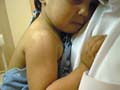
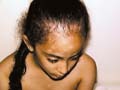
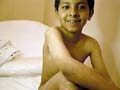
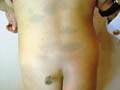
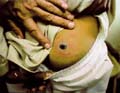
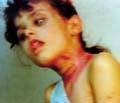
Pigmentation occurs in about 10% of patients with primary thyrotoxicosis. It is usually diffuse and is broadly of Addisonian pattern but involvement of the mucous membranes is uncommon and pigmentation of nipples and genital skin is less striking. Clinical Manifestations Diffuse pigmentation appears at birth in the infant of a thyrotoxic mother. The eyelids are occasionally conspicuously pigmented. Some patients show chloasmal rather than diffuse pigmentation. The incidence of vitiligo is increased. Hypermelanosis associated with other diseases. Increased pigmentation is an unconstant feature of a wide variety of systemic disorders and may be associated with malignant disease. Chronic infections: Pigmentation may occur in many chronic infections, but it is difficult to determine the relative responsibility of the infection, malnutrition and other factors. It has been suggested that the activity of the reticulo-endothelia system (RES) is inversely related to adrenocortical activity. With the stimulation of the RES by these chronic infections and a consequent reduced adrenocortical activity, this would lead to an enhanced pigmentation of the skin. Systemic infections: Hyperpigmentation may accompany certain chronic infections as malaria, kala-azar, schistosomiasis and tuberculosis. Subacute bacterial endocarditis: Diffuse light-brown pigmentation is an indication of the later stages of sub acute bacterial endocarditis. Skin diseases associated with hyperpigmentation: Different skin diseases may be associated with hypermelanosis. Some of these include the following: Discoid lupus erythematosus. Lichen planus. Chronic eczema. Dermatitis herpetiformis. Porphyia. Chronic photodermatitis and long time exposure to direct sunlight. Malabsorption diseases such as Gaucher‘s and Niemenn-Pick disease. Neoplastic diseases. In cachectic states, there may be diffuse hyperpigmentation of the skin as in Addison‘s disease. In the ectopic ACTH syndrome, which may occur in patients with oat-cell carcinoma of the bronchus, pigmentation is usual. The tumor has been shown to produce a distinct MSH-like compound. In an adult, acanthosis nigricans is associated usually with an internal malignancy, almost invariably an adenocarcinoma. The hypermelanosis affects the axillae, nipples and umbilicus, which also show a warty papillomatosis. These skin changes may later become generalized. The mucous membranes are frequently involved. A diffuse dermal melanosis, having a slaty-blue color, can occur secondary to melanoma. Pigmentation is an uncommon manifestation of the lymphomas, occurring in 10% of cases of Hodgkin‘s disease and in 1 or 2% of cases of lymphosarcoma and lymphatic leukemia. The pigmentation is of Addisonian type, but without involvement of the mucous membranes. Malnutrition: may be a factor in increasing skin pigmentation. Skin excoriation and scratching due to chronic skin itchy diseases. Mycosis fungoides May cause diffuse progressive hyperpigmentation. Cytostatic drugs used for the treatment of these disorders also can produce increased pigmentation of the skin. Diseases of the nervous system. Diseases of the nervous system particularly those involving the diencephalon and the substantia nigra show pigmentations of the addisonian type. Intense pigmentation is a feature of Schilder‘s disease but some increase in pigmentation is not uncommon in schizophrenia, emotional stress, Parkinsonism, hepatolenticular degeneration and in ependymomas. Asthma Asthmatic attacks may be preceded by 3 or 4 days of diffuse darkening of the skin. Collagen diseases Generalized pigmentation in scleroderma may be intense and diffuse or of Addisonian type, but without mucous membrane involvement. Pigmentation may be a conspicuous feature of morphoea and may indeed be the presenting symptom. Dermatomyositis and systemic lupus erythematosus may be accompanied by hyperpigmentation. Pigmentation is usually generalized occasionally observed in rheumatoid arthritis and Still‘s disease. Renal failure Chronic renal disease with nitrogen retention is frequently accompanied by increased pigmentation. Anemia Hyperpigmentation of the skin occurs in vitamin B12 deficiency and is more common in dark-skinned races . The pigmentation often has a rather dappled and mottled appearance, and particularly affects the face, hands and feet. Sometimes only the fingers are affected. Treatment with vitamin B12 reverses the pigmentation of the skin back to normal. In pernicious anemia there is an increased incidence of vitiligo and of premature grayness of the hair. In the hemolytic anemia, hypermelanosis and hemosiderosis may develop on the lower legs. Hemochromatosis Pigmentation, bronzed or slaty-gray in color, involves first the exposed skin but later it is generalized. It is present in some 90% of cases, but may not be conspicuous. The diagnosis should be suspected when pigmentation of this pattern occurs in middle-aged men, and will be supported by the finding of an enlarged liver and diabetes and confirmed by the high level of serum iron. Hepatic cirrhosis A diffuse hypermelanosis is seen in patients with liver cirrhosis. Lichen planus may be associated with primary biliary cirrhosis and as lesions resolve they leave slaty-brown hypermelanotic macules. Amyloidosis Localized pigmentation, often symmetrical, is seen in both lichen and macular amyloidosis. The macular type of amyloidosis is often mistaken for post inflammatory hyperpigmentation, but the lesions often have a distinctive “ripple“ pattern and microscopic studies will reveal the presence of amyloid. Melanophages are to be found in the papillary dermis. The melanin contained in these dermal cells is derived from degenerating basal keratinocytes and melanocytes. Vitamin deficiency In severe deficiency of vitamin A, generalized pigmentation may occur, as well as pigmentation of the horny follicular papules. Conjunctiva pigmentation has been noted particularly in the Mongoloid races and may be striking, especially in the bulbar conjunctiva. Vitamin B12 and folate deficiency Vitamin B12 and folate deficiency show diffuse, brown pigmentation is seen occasionally in patients with folic acid deficiency and also in vitamin B12 deficiency. Pigmentation of the fingertips and nails of a patient with B12 deficiency is reported. Pellagra Pigmentation is frequent in pellagra. It is often of Addisonian type but the pigmentation is accentuated on the face and hands and on areas of friction or pressure, or as a sequel to pellagrous dermatitis. Drugs Cloropromazine: causes purple pigmentation or slate colored pigmentation. Chloroquine: causes bluish black hyperpigmentation Estrogens: causes skin melanosis Other drugs such as phenothiazine, antipyrine, tetracycline cause skin pigmentation Bismuth: causes gum hyperpgimentation Silver: causes metallic blue pigmentation Scurvy Deficiency of ascorbic acid is sometimes associated with pigmentation, which is usually of Addisonian type. Malabsorption syndromes In sprue and other malabsorption syndromes pigmentation is of common occurrence. It may be of Addisonian type but without involvement of the mucous membranes, or may occur in well-defined patches on the face and neck and occasionally on the trunk. The scaly inflammatory plaques, which may develop in these syndromes, are usually followed by intense pigmentation. Vagabonds‘ disease Vagabonds‘ disease classically occurs in those in whom lack of food is combined with lack of cleanliness, and heavy infestation with pediculi. The pigmentation is basically of Addisonian pattern and the mucous membranes may be involved. Hypermelanosis is probably post inflammatory and related to the scratching from the pediculosis infestation. Areas of hypomelanosis occur and there is a decrease in the number of melanocytes, which show degenerative changes. Post-inflammatory hypermelanosis. Hypermelanosis commonly follows acute or chronic inflammatory processes in the skin as in lichen planus, herpes zoster, dermatitis herpetiformis, papular urticaria and collagen diseases. The intensity and persistence of the hypermelanosis are greater in dark-skinned subjects. Post-inflammatory hyperpigmentation may occur following trauma to the skin. It can occur following dermabrasion and particularly in those who are racially pigmented, of skin type 111 & 1V. Collagen diseases: such as discoid lupus erythematosus. Congenital heart disease: hyperpigmentation particularly central cyanosis occurs secondary to congenital heart disease.
Pityriasis alba is a non-specific dermatitis of unknown origin that characteristically produces erythematous scaly patches, which subsides to leave areas of depigmentation. Etiology The exact cause of pityriasis alba is not clear. We found that there are different predisposing factors for pityriasis alb.These include the following: Over exposure to sunlight. Children spending long time in the swimming pools containing excess chlorine and later long exposure to direct sunlight. Gastro- intestinal disturbances. Fevers and recurrent infections such as chronic tonsillitis. Obese children are more susceptible. Over eating especially at night and sleeping immediately after the night meal. Excess cosmetics and topical preparations. Psychic, hyperactive and irritable children. Clinical Features Pityriasis alba is usually a chronic skin eruption affecting predominantly children. The clinical picture of the skin lesions varies from scaly erythematous patches to depigmented plaques. The site involved is mainly the face . Other sites like shoulders and the limbs may be affected but to a lesser extent. The skin lesion is usually asymptomatic and the patients usually seek advice due to cosmetic changes, due to the depigmentation at the site of the lesion. The course is variable; healing of the patches may take few days or weeks and may last for more than a year. Remission and relapse of the skin lesions are not uncommon, where the new patches may appear at the previous sites. Diagnosis Diagnosis of pityriasis alba depends on the clinical picture . The skin lesions appear as erythematous, well demarcated covered by fine, branny adherent scales or as a depigmented patches of a chronic course eliciting minimal symptoms and affecting mainly children. Differential Diagnosis Psoriasis It is not difficult to differentiate pityriasis alba from psoriasis, where the latter patches show more dense silvery scales and may involve the scalp and the extremities which are not the sites for pityriasis alba. Vitilligo In vitilligo the patches are more depigmented and whitish in color and may affect extensive areas and if the scalp is involved the affected area shows usually grayish hair. Discoid eczema Discoid eczema may simulate pityriasis alba clinically but the lesion of discoid eczema is erythematous showing some vesiculation in the acute stage, while in the chronic cases of discoid eczema the dry scaly lesions, especially when it is solitary on the face may simulate pityriasis alba. The symptoms such as itching are more in discoid eczema. The lesion of discoid eczema and the skin changes are more prominent. Treatment Treatment of pityriasis alba is non-specific. Emollient creams may be helpful. Mild steroid such as hydrocortisone combined with salicylic acid, vioform or tar is some times helpful. 15% oil of pergamount applied in the morning may help repigmentation.
ERYTHEMA
DYSCHRONICUM PERSTANS This is a rare syndrome of unknown etiology that affects children and old age. The disease simulates lichen planus clinically and histologically. . It is sometimes called lichen pigmentosus. The exact relationship to lichen planus is uncertain but there are close similarities and both conditions may coexist. Clinical Manifestations Skin lesions manifest with numerous macules of varying colors, of gray with a red, slightly raised and palpably infiltrated margin. They vary in size and tend to coalesce over extensive areas of the trunk, limbs and face. Against the general grayish background are macules of hypomelanosis or hypermelanosis. Histopathology Vacuolar degeneration of the basal layer. The epidermis contains much pigment and there is pigmentary incontinence. The dermal vessels are sleeved with an infiltrate of lymphocytes and histiocytes, and there are many melanophages. Differential Diagnosis The pigmented macules resemble very closely the lesions of the late pinta, but the negative dark-field examinations; negative serological tests for syphilis and lack of response to penicillin are important features.
Etiology Genetic and racial factors Dark skin individuals especially type III & IV skin types, are more susceptible to hypermelanosis. Mongoloids. Endocrine factors play a major role in cloasma and are implicated to some degree in other melanosis. Addisonian pigmentation, the condition is more frequent in women. External agents: light and photodynamic chemicals are essential factors. Occupational melanosis. Riehl‘s melanosis, erythrosis and poikiloderma of Civatte. Cosmetics are frequently a cause of facial melanosis. Tar derivatives and compounds in cosmetics. Nutritional: and other factors may be involved. Some cases occur in children and in others there is no history of contact with cosmetics. Idiopathic : certain types of facial melanosis may be of unknown cause. Clinical Features Brownish-gray pigmentation develops quite rapidly over the greater part of the face but is more intense on the forehead and temples. Smaller pigmented macules, often perifollicular, lie beyond the indefinite margin. The pigmentation may extend to the chest, neck and scalp, and occasionally involves hands and forearms. Horny plugs fill the follicles and there may be some scaling.
This is hyperpigmentation that usually involves the periorbital areas and may extend to the eyelids and cheeks .The pigmented areas are either deep brown or appear as black halos around the orbits. Etiology Different factors may predispose to periorbital hyperpigmentation mainly: - Sleep disturbance. - Exhaustion to the periorbital muscles as a result of reading, TV and others. - Stress and emotional instability. Treatment of Periorbital Melanosis Cold milk compresses applied to the periorbital area twice daily followed by a bleaching agent such as hydroquinone (Eldoquine 2%).
This skin disorder occurs in middle-aged women on the sun-exposed areas. The age incidence suggests that an unknown endocrine factor or age change may also play some part. Clinical Features Reddish-brown reticulate pigmentation with telangiectasia and atrophy develops in irregular more or less symmetrical patches on the lateral cheeks and the sides of neck but spares the area shaded by the chin.
NEVUS OF OTA
The color is variable, but usually is either slate-brown or blue. The sclera is involved and there may be hyperpigmentation of the cornea, iris, retina, ocular muscles and orbit. Sometimes there is pigmentation of the hard palate. Deafness occurring in a patient with nevus of Ota has been reported. Melanomas may develop in the skin, eye and brain of these patients, more frequently in white patients than in the Japanese.
POIKELODERMA
CONGENITALE Poikeloderma congenitale is a rare disease, appears early in infants usually in the first six months after birth, characterized by telengectasia, atrophy, and skin pigmentation. Clinical Features Skin lesions The skin lesions appear first on the face and hands and extend to neck and trunk where, the lesions at the end stage closely resemble chronic radiodermatitis.
Photosensitivity Light sensitivity is a feature of many cases. Light sensitivity may be so severe provoking a bullous response that tends to diminish after early childhood; it may persist into adult life. Keratoses: keratoses develop on exposed skin from adolescence onwards presenting with large warty keratoses of hands, wrists, feet and ankles. Squamous carcinoma may develop in the keratoses or in the surrounding atrophic skin. Hair: Eyebrows and lashes, pubic and axillary hair is often sparse or absent. Scalp hair is often sparse, fine and may be absent. Nails: are normal or small and dystrophic. Teeth: are often normal, but microdontia and early caries have been reported. Eyes: bilateral cataracts have developed, usually between the fourth and seventh year. Physical development: is frequently retarded; most patients are of small stature and some are dwarfs. The dwarfism is proportionate with slender, delicate limbs, small hands, feet, and short stubby fingers. The skull may be small and the features bird-like, sometimes with a saddle-nose. Expectation of life appears to be normal. Endocrine abnormalities: Hypogonadism of slight or severe degree is frequent. Hyper parathyroidism appears also to be increased. Mental development is usually normal, but some cases may be retarded. Diagnosis of Poikeloderma The essential features in differential diagnosis are: The age of onset. The distribution of the lesions. The combination of atrophy, telangiectasia and mottled pigmentation is more intense on light-exposed skin. Differential Diagnosis Werner‘s syndrome: The skin changes are essentially sclerodermatous, and both skin and ocular lesions develop later than in the Rothmund-Thomson syndrome. Dyskeratosis congenita: Reticulate pigmentation develops between the ages of 5 and 13, and is most marked on the neck, trunk and thighs. Atrophy and telangiectasia may appear later. The nail changes are constant and severe. Progeria the child is often small but otherwise normal during the first year, thereafter development is retarded. The scalp hair, brows and lashes are lost and the skin assumes an increasingly senile appearance. Cockayne‘s syndrome: Light sensitivity is a conspicuous feature after the first year, but there is no poikiloderma. Hypohidrotic ectodermal dysplasia: There is combinations of conical teeth, hypotrichosis and partial or complete anhidrosis. The skin is atrophic but not poikilodermatous. Xeroderma pigmentosum: In the mild forms there is only freckle-like macules. Rothmund-Thomson syndrome: Telangiectasia is the conspicuous feature. Focal dermal hypoplasia: Telangiectasia is often irregular, linear and present at birth. Bloom‘s syndrome: Eerythema, and not poikiloderma, is the essential change.
This syndrome, probably is determined by an autosomal dominant gene Clinical features
Treatment of Hypermelanosis Treatment of the cause. Avoid excessive exposure to sun. Sunscreens can be used before exposure to sunlight. Cosmetic camouflage may also be indicated. Skin bleaching agents Skin-bleaching preparation: hydroquinone (Eldoquine 2-4%) may help in some cases. Care should be taken in using strong concentrations especially in children and those with delicate skin. Local irritation is very common and hypopigmentation, pigmented colloid milium and even vitilligo like reactions may develop with the long use of this potent hydroquinone. Side effects of hydroquinone It should be noted that hydroquinones are not very effective for post inflammatory hyperpigmentation. The monobenzyl ether of hydroquinone is responsible for many therapeutic and cosmetic disasters and the compound should be used only to bleach away the remaining pigmented areas in patients with extensive vitiligo. Several other substituted phenols such as 4-isopropylcatechol can produce cutaneous depigmentation: however, this compound and others are irritant and may produce local sensitization. Lasers are recently used effectively and safely in treatment of pigmented skin lesions. Q-switched ruby, Pulsed dye laser and other types can be used with variable degrees of improvement. The results are better in those who have fair or white skin color.
|
| Contents | << Previous Chapter | Next Chapter >> | Search |